The State of Lung Imaging
Current lung diagnostics and imaging modalities are limited in their ability to provide detailed visibility to lung function, and some require patients to be exposed to radiation.
Learn About Xenon MRI
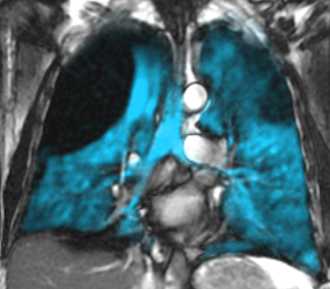
Xenon MRI is an evolution in lung imaging technology, providing a direct measure of regional lung function in a single 10-to-15-second breath-hold.
Our Mission: Helping Millions of Patients with Chronic Lung Disease
It’s estimated that almost 550 million people worldwide1 are living with chronic lung disease. Polarean believes that providing an enhanced picture of the lungs may help a variety of patients suffering from lung diseases such as idiopathic pulmonary fibrosis, COPD, pulmonary hypertension, radiation therapy, cystic fibrosis, asthma, and more.
Be Part of the Evolution of Lung Imaging

ABOUT XENON MRI
ONGOING RESEARCH
Polarean News
Polarean's Technology Platform
HPX Measurement Station
Provides a calibrated measurement of the polarization of hyperpolarized gas.
HPX Gas Manifold
A gas pressure regulation and purification system that allows xenon-blend cylinders, one ultra-high-purity nitrogen cylinder, and one industrial nitrogen cylinder to be connected to the HPX Hyperpolarizer.
References & Other Information:
1. Labaki WW, Han MK. “Chronic Respiratory Diseases: A Global View”. Lancet Respir Med. 2020;8(6):531-533.

